Position Your solutions at the Centre of Gene Therapy Analytical Decision Making
As gene therapy developers face growing pressure to strengthen potency assurance, improve analytical confidence, and prepare for regulatory scrutiny, analytical teams are actively seeking partners who can help them generate reliable data, reduce uncertainty, and future-proof their development strategies.
This specialist, industry led meeting brings together a highly engaged audience of senior analytical, QC, MSAT and regulatory scientists, offering your company direct access to the decision makers who are actively seeking solutions to potency assurance packages, phase-appropriate assay validation, QC readiness, raw material sourcing and cost-efficient analytical optimisation.
Through three days of in-depth technical sessions, interactive workshops, and structured networking, you will engage directly with the experts responsible for selecting analytical technologies, services, and long-term partners.

What to Expect?
Tailored Access to Analytical Decision-Makers
Meet the analytical development, QC, CMC, and regulatory scientists shaping potency, identity, and safety expectations for viral and non-viral gene therapies, and connect directly with teams evaluating technologies and partners that strengthen assay robustness, reduce variability, and support late-stage development.
Strategic Visibility in a Highly Technical Environment
Position your organisation as a trusted analytical partner through targeted marketing, curated branding, and technical speaking opportunities that place your solutions in front of a specialised audience actively seeking analytical expertise.
Stay Ahead of Analytical & Regulatory Evolution
Gain early insight into how biopharma teams are approaching potency assurance, method lifecycle management, emerging characterisation tools, and fit-for-purpose validation, and understand how analytical expectations are shifting across development phases to highlight opportunities for innovation without added regulatory risk.
Lead the Analytical Strategy Conversation
Showcase your expertise through thought-leadership presentations or expert-led panels that demonstrate how your technologies improve reproducibility, contextualise complex analytical outputs, and accelerate readiness for regulatory interactions.
Key Services & Solutions
Our attendees are looking for service and solution providers with capabilities across:
Analytical & QC Services
Supporting gene therapy developers with method development, qualification, validation, comparability, stability, and release testing across viral and non-viral modalities.
Instrument & Kit Providers
Delivering analytical instruments, platforms, and assay kits that enable high-resolution characterisation of potency, capsid composition, genome integrity, impurities, and stability
Raw Materials Suppliers
Providing critical reagents, enzymes, primers, antibodies, reference materials, and assay controls essential for consistent analytical performance, assay reproducibility, and lifecycle management in the absence of universal standards.
Contract Development & Manufacturing Organizations (CDMO/CMO)
Partnering with developers to deliver GMP-ready analytical methods, tech transfer support, and QC execution, ensuring early analytical strategies can be sustained through clinical scale-up and commercialisation.
Contract Research Organizations (CRO)
Offering flexible analytical capacity and specialised testing services to accelerate development timelines, support complex characterisation needs, and supplement internal teams during high-demand phases.
Hear What Our Past Partners Have to Say

What did you like most about this event…. 'The number of people attending and their background, that speed networking and the balance between scientific talks and time to interact with the rest of attendees'
Managing Director, Quatre Lab, 2024 Partner

I enjoyed that….'talks were good quality with good scientific content (not just sales pitches). Also good to have a gene therapy event focused on Analytical rather than a mix of analytical and manufacturing/clinical (topics)’
Associate Director of Cell & Molecular Analysis, Intertek group, 2023 Partner

'I particularly enjoyed the roundtable and panel discussions on development of potency assays, as it was interesting to hear how different companies across the industry are tackling the same issues'
Scientist, Phamaron, 2023 Partner

'Very familiar event in which I had the opportunity to approach and talk to a large number of participants'
QC Molecular Biology Specialist, Quatre Lab, 2024 Partner

'I enjoyed the small, focused conference and very relevant talks and posters'
Business Development Director, Centre for Breakthrough Medicines at Discovery Labs, 2022 Partner
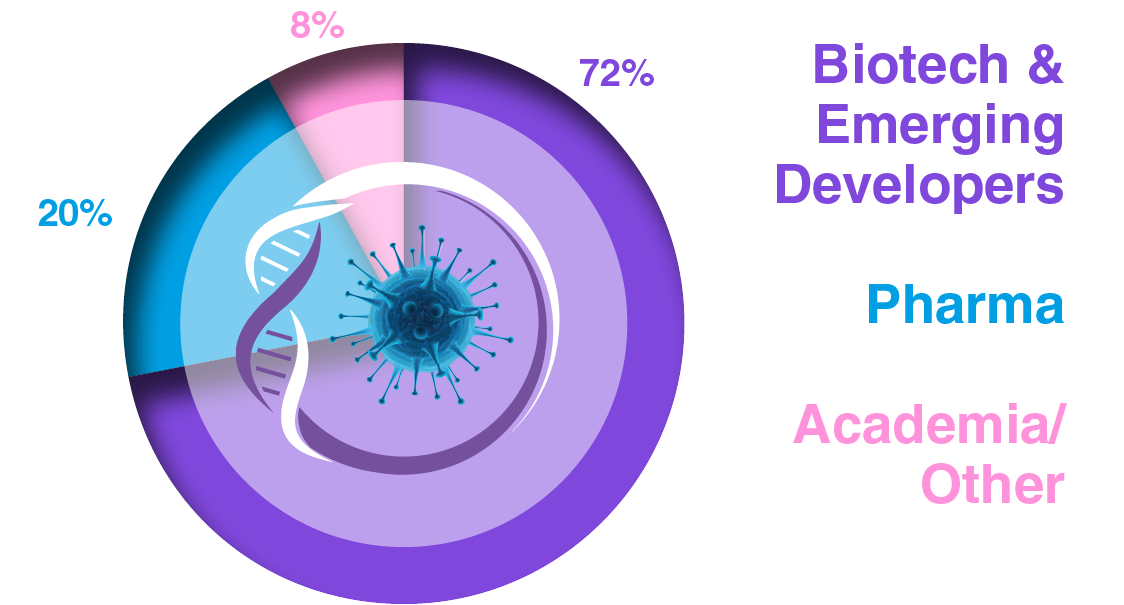
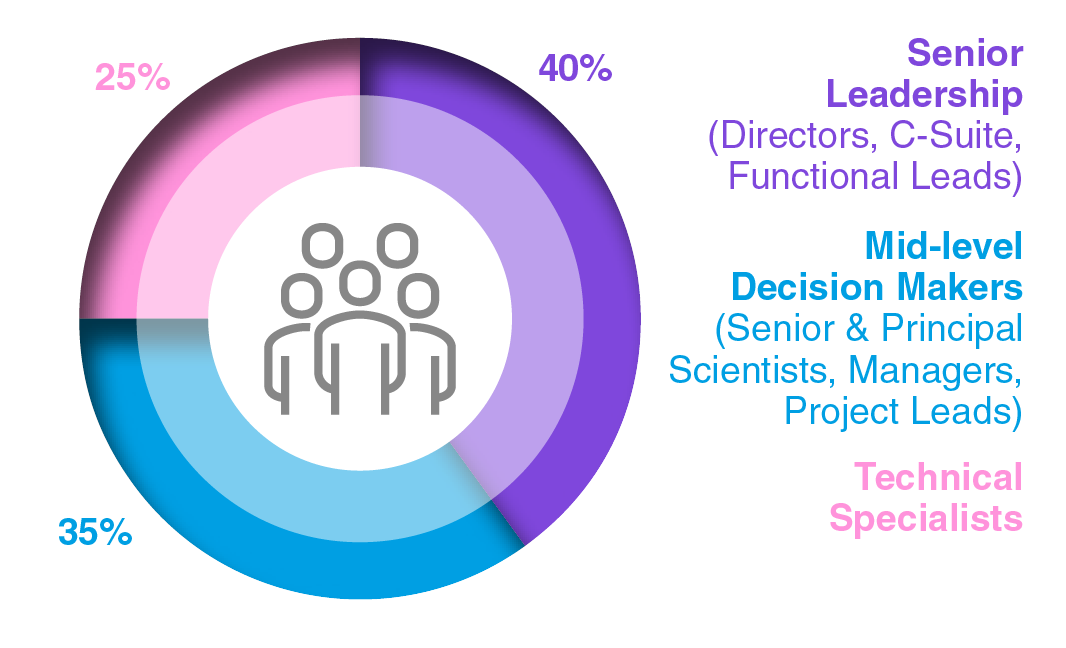
Audience Composition
Company Type
Attendee Seniority
Attending Companies Include









Available Inventory
We have a range of inventory from standard booth and speaking slots to more bespoke deliverables such as lanyards, exclusive dinners and tours to help elevate your brand.








Get in Touch
Take advantage of our bespoke sponsorship opportunities to achieve your commercial goals. Email us if you would like to get involved and discuss a bespoke package suited to your needs.

Jack Waring
Partnerships Director
Gene Therapy Analytical Development Summit Europe